In Vitro Toxicological Investigation and Risk Assessment of E
Por um escritor misterioso
Last updated 24 outubro 2024

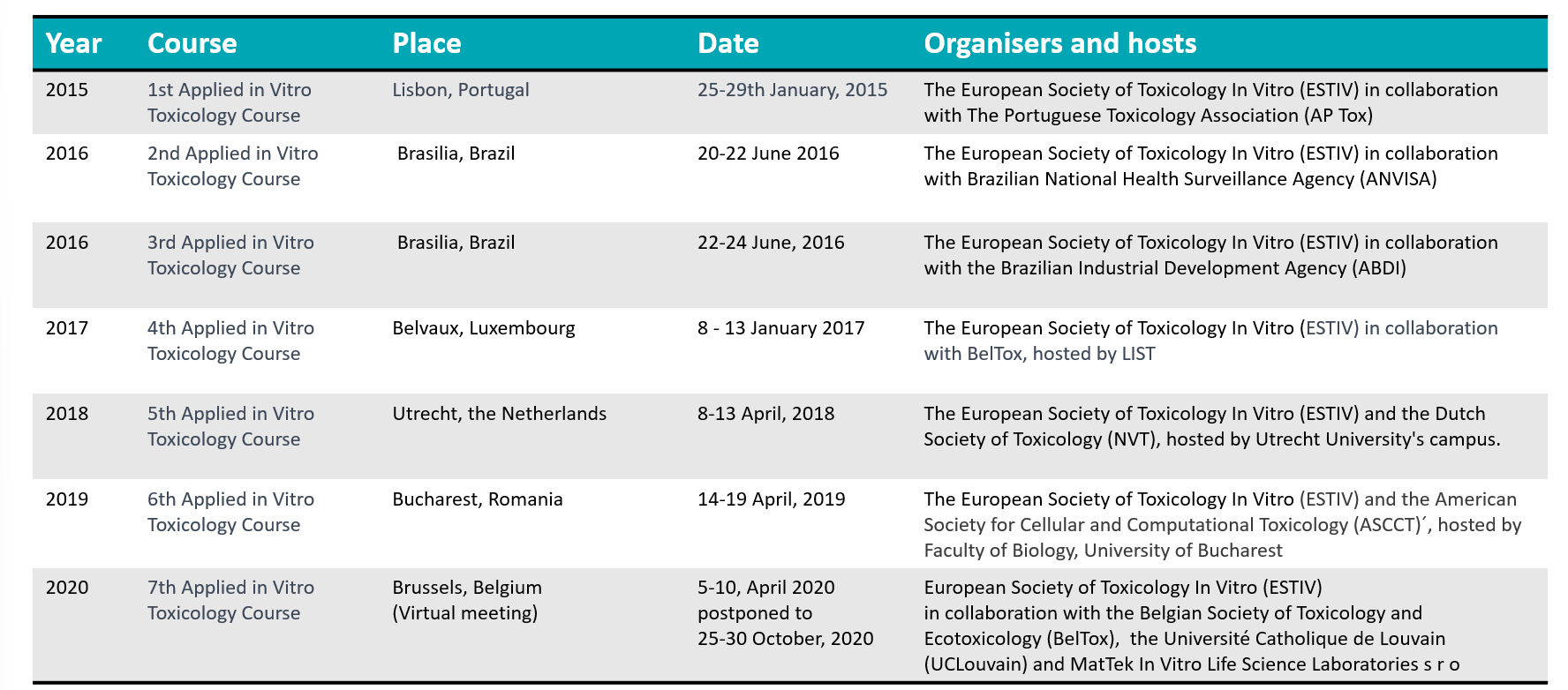
About the course European Society of Toxicology In Vitro

PDF) In Vitro Toxicology, Biodetection and Risk Assessment
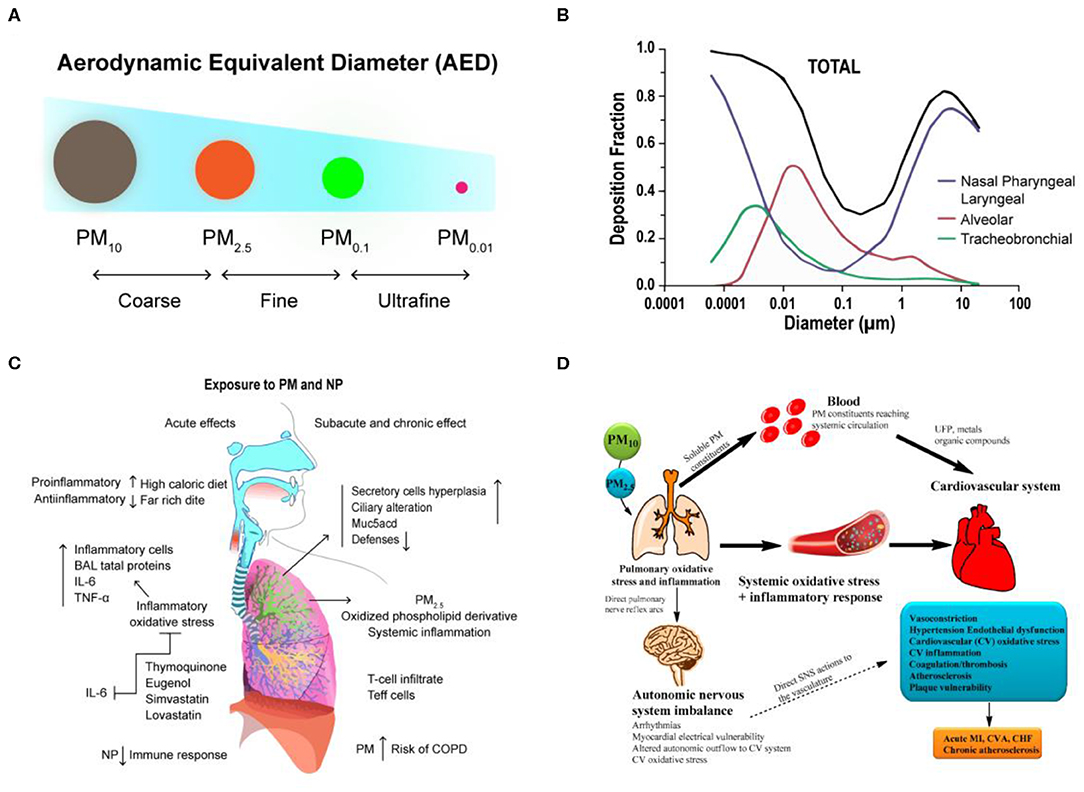
Frontiers Organ-on-a-Chip: Opportunities for Assessing the Toxicity of Particulate Matter
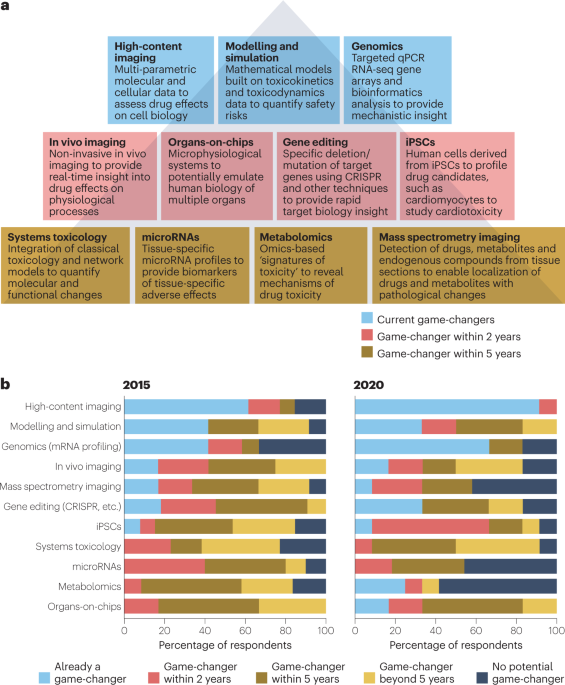
The evolving role of investigative toxicology in the pharmaceutical industry
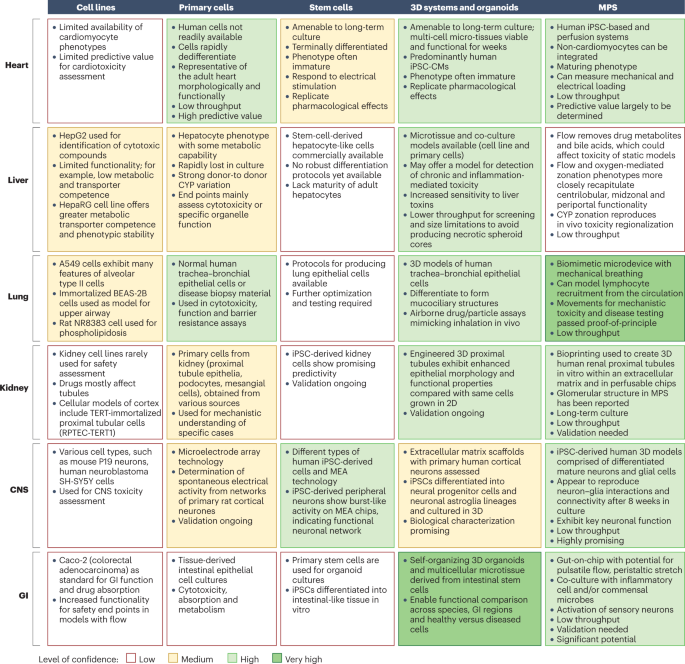
The evolving role of investigative toxicology in the pharmaceutical industry

Toxicological risk assessment and prioritization of drinking water relevant contaminants of emerging concern - ScienceDirect
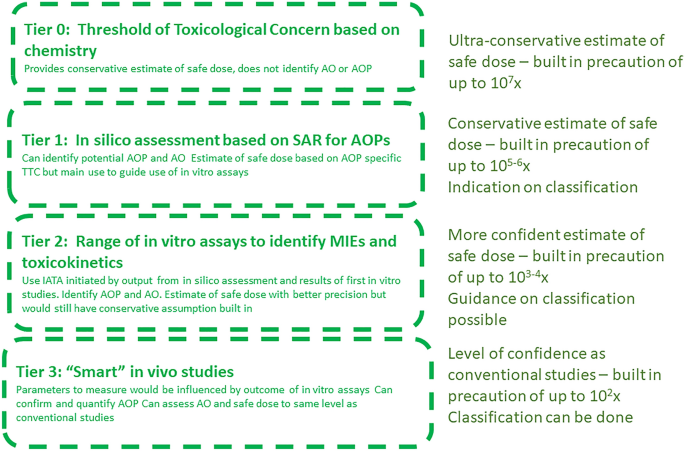
A framework for chemical safety assessment incorporating new approach methodologies within REACH
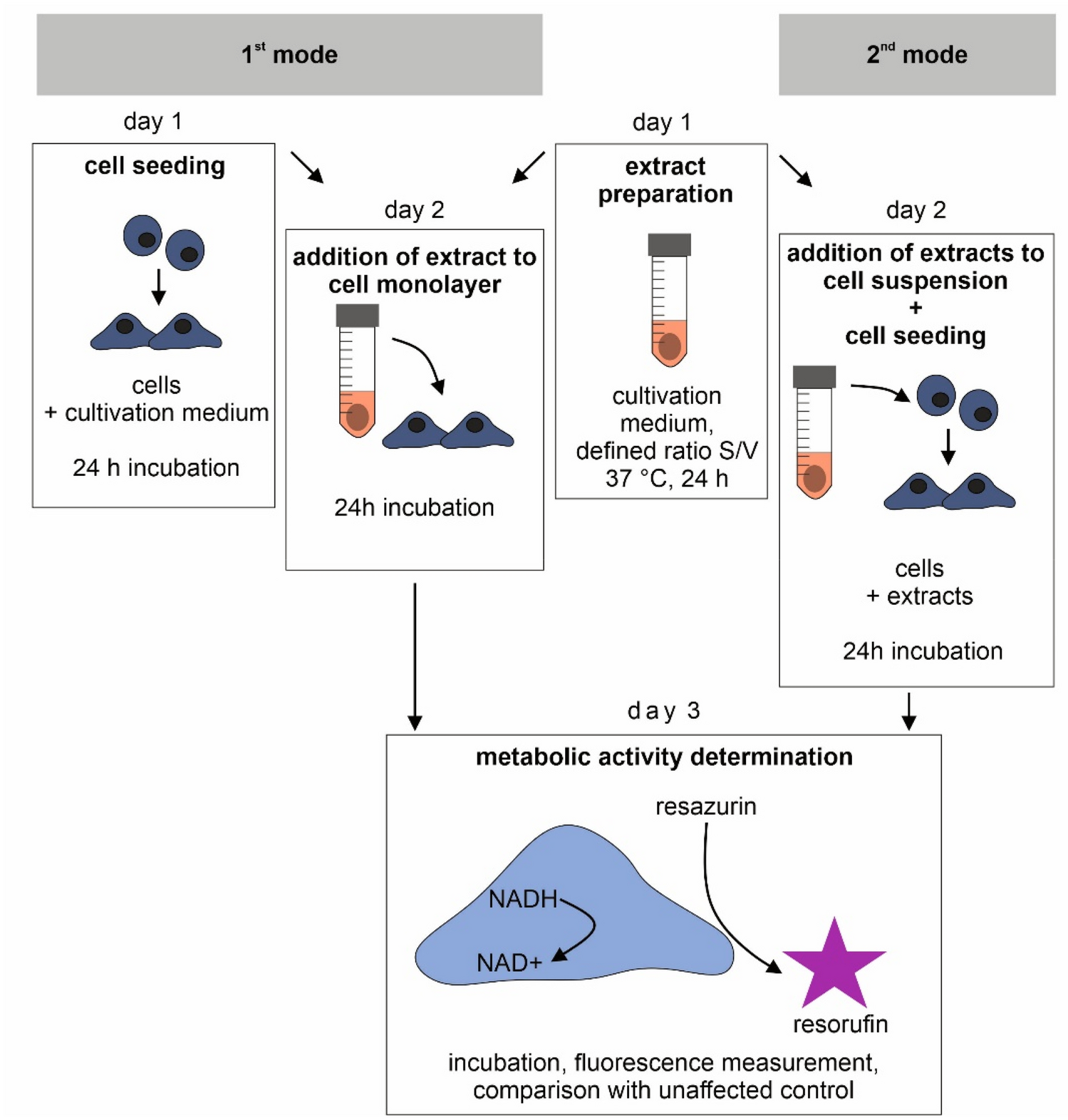
Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials
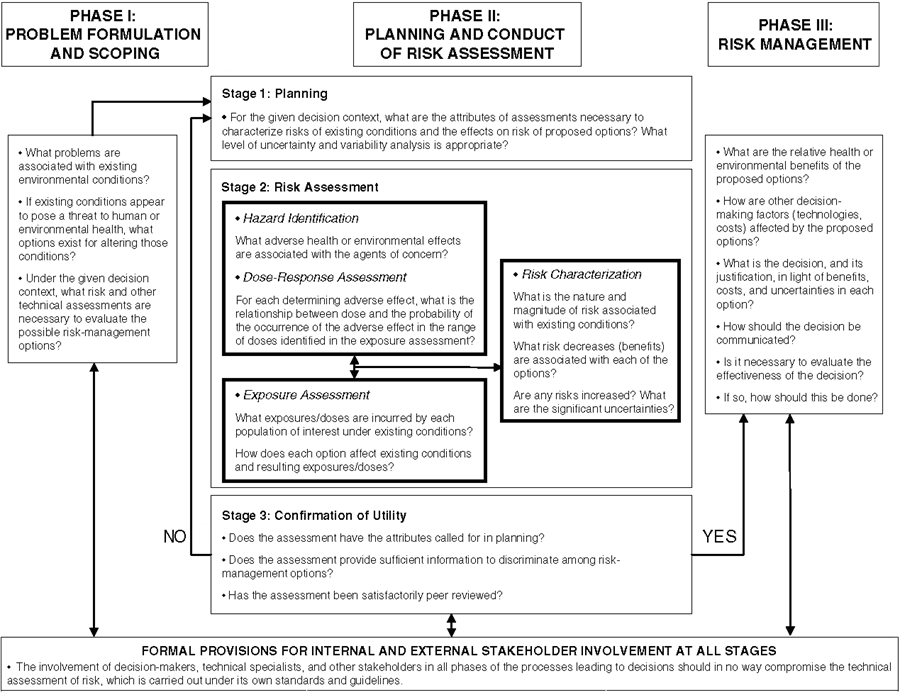
Welcome to ToxTutor - Toxicology MSDT
Toxicity Testing in the 21st Century: Defining New Risk Assessment Approaches Based on Perturbation of Intracellular Toxicity Pathways
Recomendado para você
-
 Photos: History of tobacco health claims24 outubro 2024
Photos: History of tobacco health claims24 outubro 2024 -
Übbs Pouches24 outubro 2024
-
 JPS Players launches Crushball cigarillo for menthol smokers24 outubro 2024
JPS Players launches Crushball cigarillo for menthol smokers24 outubro 2024 -
Player's, Player's Smooth and Player's Original, Regular Size, 20 and 25 cigarettes recalled due to Fire Hazard - Canada.ca24 outubro 2024
-
 Players Select Blend 100's Lights, 25-pack – vintage American Cigarette Pack24 outubro 2024
Players Select Blend 100's Lights, 25-pack – vintage American Cigarette Pack24 outubro 2024 -
What's the strongest cigarette out there? - Quora24 outubro 2024
-
 PUFF MAX Disposable Device (5000 Puffs)24 outubro 2024
PUFF MAX Disposable Device (5000 Puffs)24 outubro 2024 -
 POM Pepper Spray in Self Defense Tools24 outubro 2024
POM Pepper Spray in Self Defense Tools24 outubro 2024 -
Zacks Industry Outlook Highlights Philip Morris, Altria Group and 22nd Century24 outubro 2024
-
 Imperial Tobacco launches JPS Players Easy Rolling Tobacco, Product News24 outubro 2024
Imperial Tobacco launches JPS Players Easy Rolling Tobacco, Product News24 outubro 2024
você pode gostar
-
 7 Everyday English Idioms and Where They Come From24 outubro 2024
7 Everyday English Idioms and Where They Come From24 outubro 2024 -
 OpenAI Says Stonemasons Should Be Fine in the Brave New World24 outubro 2024
OpenAI Says Stonemasons Should Be Fine in the Brave New World24 outubro 2024 -
 Pool Party Backdrop Summer Beach Pool Party Decorations24 outubro 2024
Pool Party Backdrop Summer Beach Pool Party Decorations24 outubro 2024 -
 Every Streamer That Raised Prices in 2023: Netflix, Shudder, and More – IndieWire24 outubro 2024
Every Streamer That Raised Prices in 2023: Netflix, Shudder, and More – IndieWire24 outubro 2024 -
 Fan complaints seem to have changed Cammy's Street Fighter V look24 outubro 2024
Fan complaints seem to have changed Cammy's Street Fighter V look24 outubro 2024 -
 Animes, onde assistir? Noragami \(^ヮ^)/ Amino24 outubro 2024
Animes, onde assistir? Noragami \(^ヮ^)/ Amino24 outubro 2024 -
Will Chopper ever get another Devil Fruit power in 'One Piece'? If24 outubro 2024
-
 Fortaleza x Cruzeiro ao vivo: como assistir online e transmissão24 outubro 2024
Fortaleza x Cruzeiro ao vivo: como assistir online e transmissão24 outubro 2024 -
 Magazine Luiza fará evento especial para lançamento de God of War24 outubro 2024
Magazine Luiza fará evento especial para lançamento de God of War24 outubro 2024 -
 Reshiram & Zekrom GX (Tag Team) - 259/236 Hyper Secret Rainbow24 outubro 2024
Reshiram & Zekrom GX (Tag Team) - 259/236 Hyper Secret Rainbow24 outubro 2024

